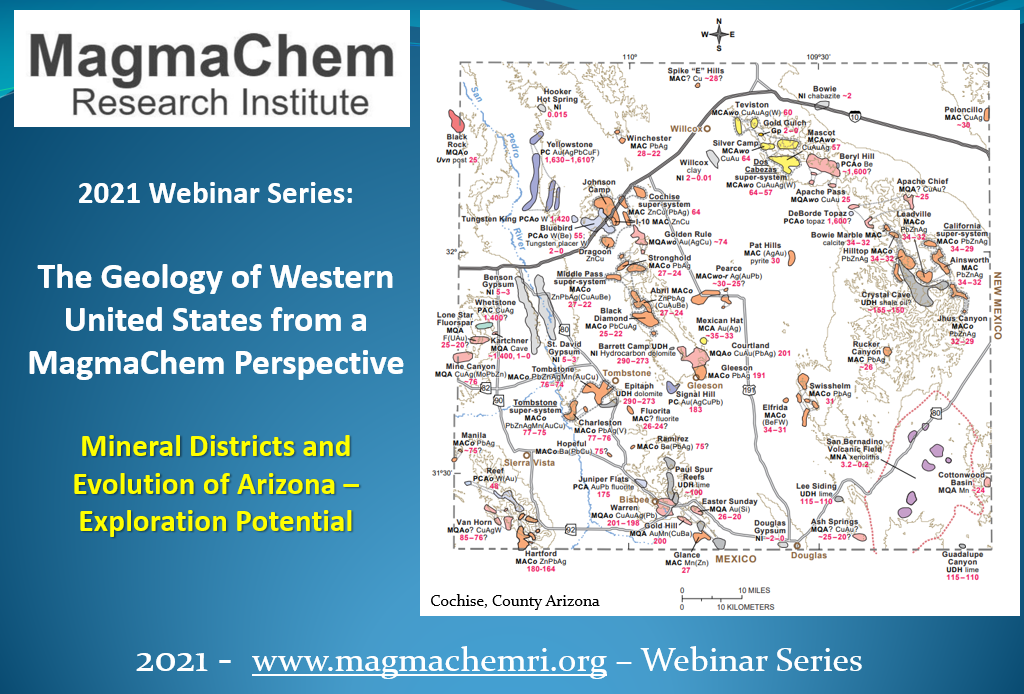
Publishing the Mineralogy of Arizona
Years of hard work have gone into understanding every known mineral in the state of Arizona. MagmaChem is focused on the publication of this book, and we plan to give several talks and presentations with the co-authors later this year. STAY TUNED!!